EZ¹ successfully completes Customer Evaluation phase.

Endoscopic removal of colonic polyps and early cancers eliminates the need for surgical resection and possible temporary or permanent colostomy.
Lumendi, LLC has received U.S. Food and Drug Administration (FDA) 510(k) clearance for DiLumen EZ¹, a single-use, disposable endotherapy device for endoscopic mucosal resections (EMR) and difficult colonoscopies.
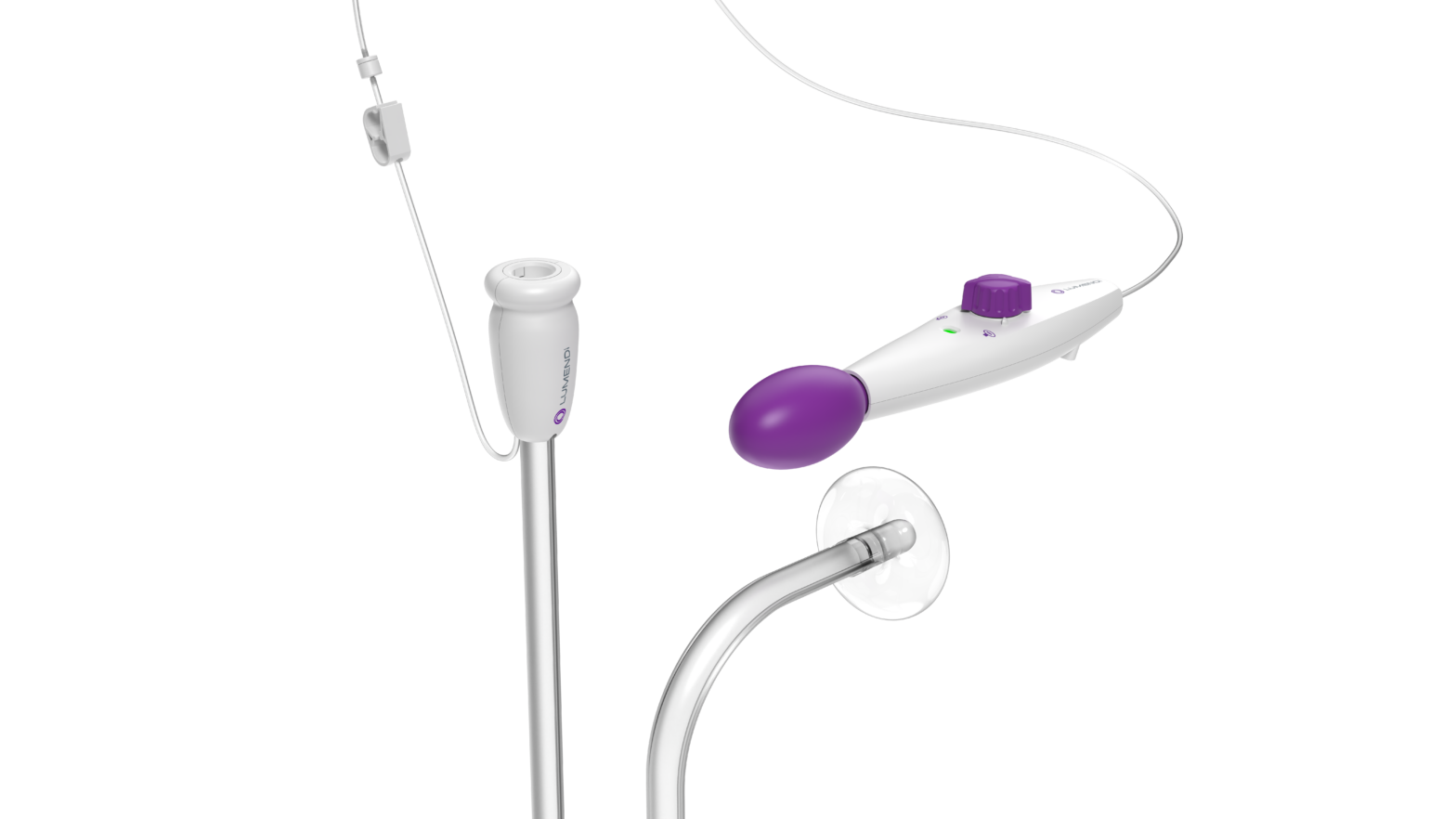
DiLumen EZ¹ The DiLumen EZ¹ is a variant of the DiLumen design, specially intended for the removal of complex polyps using the EMR* technique. Thanks to its unique balloon design,… Continue reading Expansion of the Dilumen Platform


Since the company’s inception, we have accumulated over 100 DiLumen users worldwide. Half of these physicians have begun using our devices in the last 12 to 18 months. This year’s DDW was our first opportunity in three years to present Lumendi and our significantly enhanced DiLumen platform to users and industry.
“The new DiLumen EZ Glide significantly improved our ability to remove four cecal polyps in a recent case. We could not have ever done this without it.” Dr. Stuart Gordon… Continue reading Dr. Stuart Gordon
“EZ Glide represents a huge improvement in usability of DiLumen technology. The enhanced ease of use has prompted me to use DiLumen more often for complex colonic polypectomies.” Dr. David… Continue reading Geisinger Medical Center, Danville, PA

DiLumen EZ-Glide improves visibility and simplifies intraoperative



Thank you for supporting our Patient Awareness Initiative



Many studies have documented that an endoscopic approach for ESD is a safer and less expensive alternative to removing these polyps, leading to fewer postoperative complications and shorter hospital stays.

Superiority of DiLumen over traditional (non-overtube) ESD for complex colorectal polypectomy.
Removing colorectal polyps and early cancers by endoscope eliminates the need for surgical resection and the potential for colostomy. This study reports clear improvements in safety, dissection time, dissection speed, R0 resection rates (clean pathological margins), and navigation time over non-DiLumen-assisted (freehand) procedures.
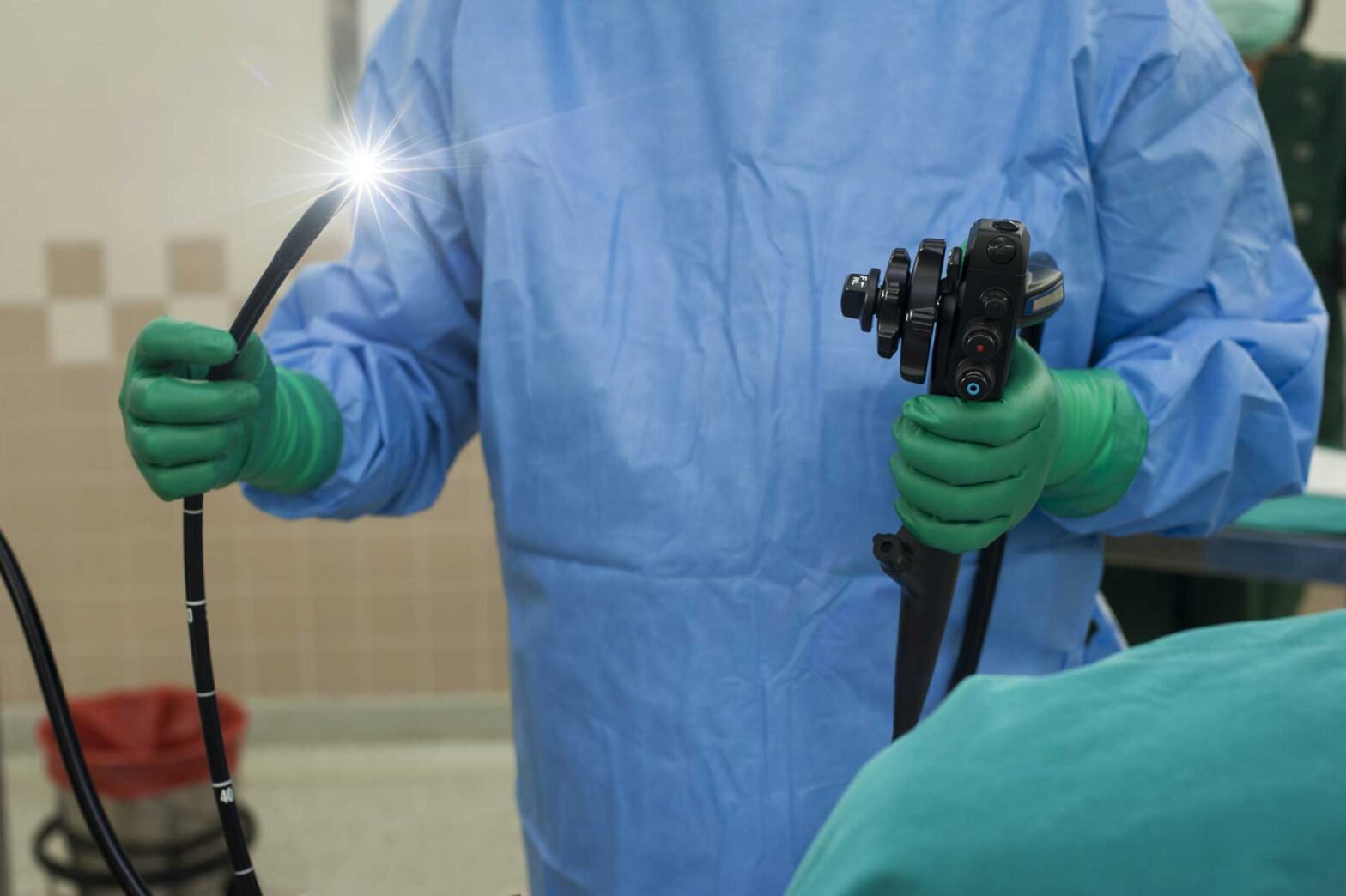
The Cost-Effectiveness of DiLumenTM for Endoscopic Intervention of Complex Colonic Polyps
James White, D.O., VP of Medical Affairs, Lumendi
Karl Florence, Reimbursement Consultant.
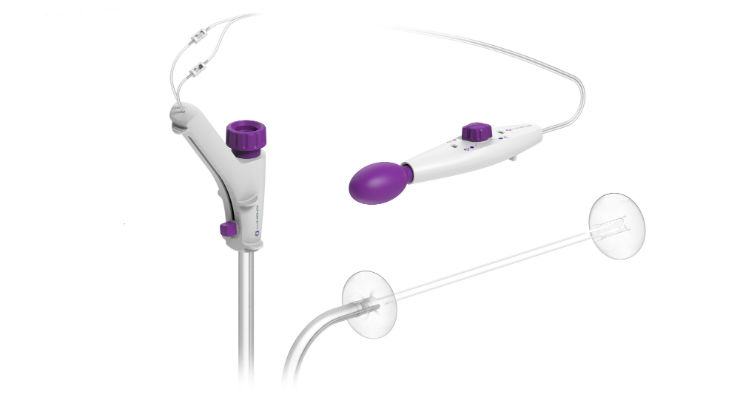
Pioneering Endosurgery reflects Lumendi’s innovative, three-part pathway to:
expand indication areas and procedures in the upper gastrointestinal tract, which includes a recent submission to the FDA;
innovate improvements planned for the DiLumen EIP, including a proprietary lubricated inner surface of DiLumen’s sheath for better endoscope handling performance; and
commercially release the DiLumen C2 platform[1] to bring robotic-like end-effectors to endolumenal surgery in a low-cost, disposable format to provide endoscopists with unprecedented control of tissue.
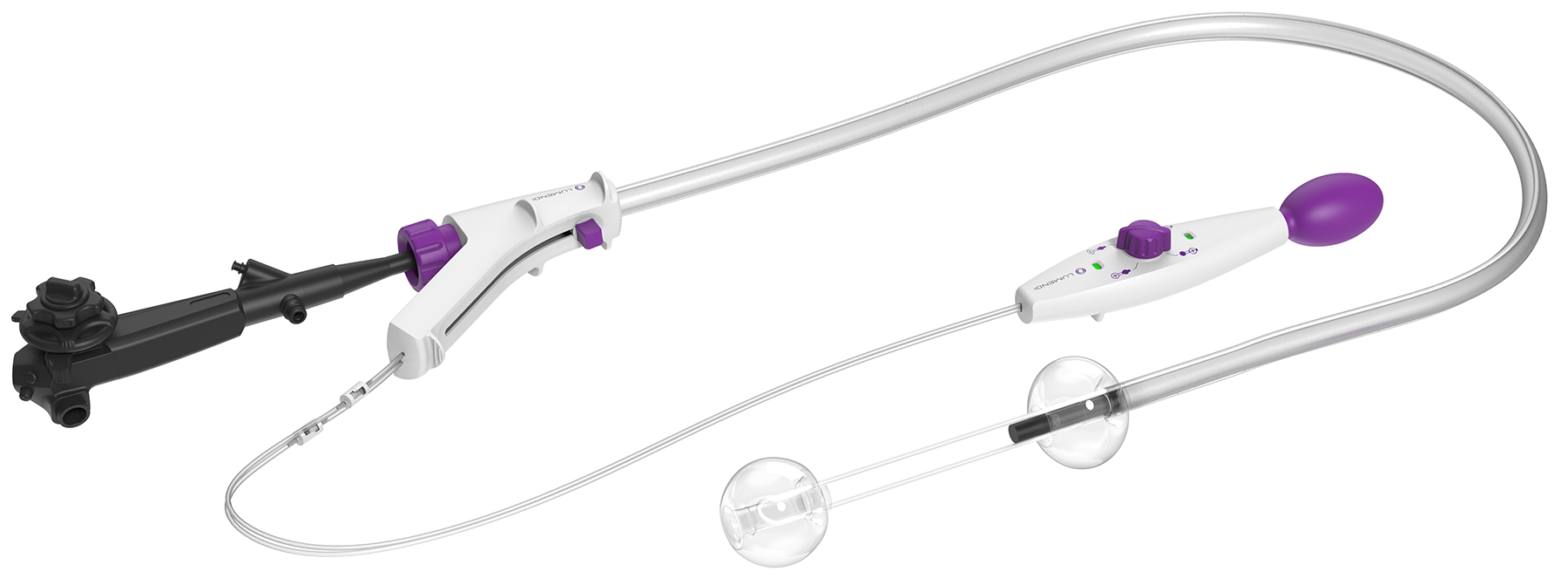
A study evaluating endoscopic procedure time removing complex colorectal polyps shows superiority of Lumendi’s DiLumen device over a control group.