Company profile
Founded in December 2014, Lumendi develops devices that enable less invasive endoluminal procedures in the GI tract, primarily in the colon. These devices have the potential to replace many invasive laparoscopic and open surgical procedures performed in hospital settings.
Our team
Our technology
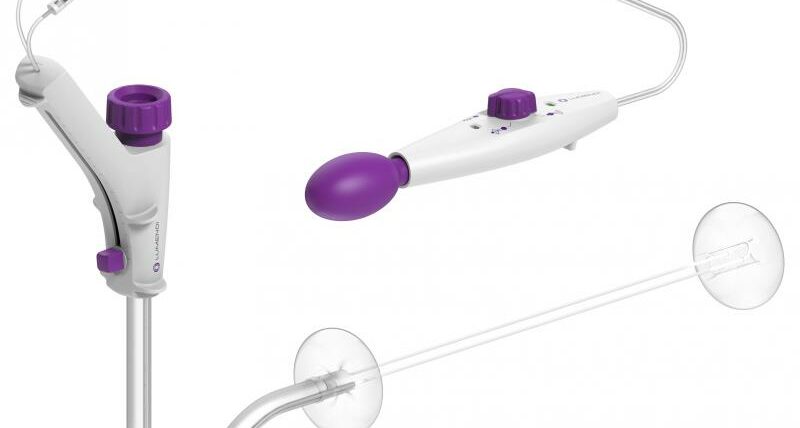
Our innovative DiLumen® Endoluminal Interventional Platform (EIP) technology transformed the endosurgery landscape.
DiLumen works with your existing endoscopic equipment to enable endoluminal therapeutic procedures such as complex polypectomies.
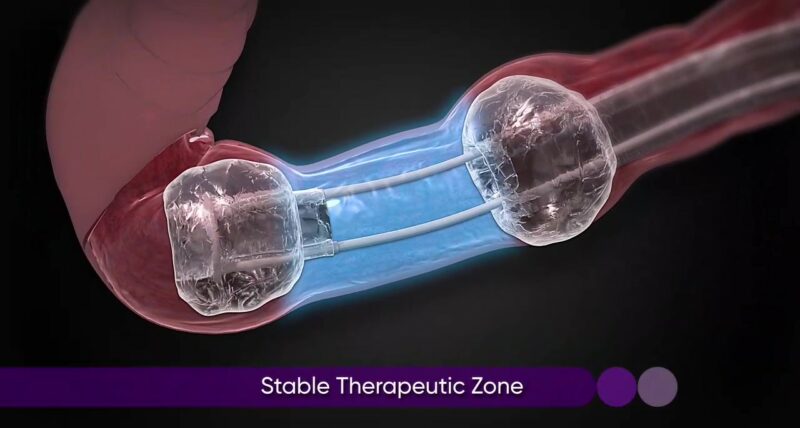
Its two balloons create a stabilized area within the GI tract, improving control and tissue manipulation in the rectum and colon.
Our clinical advisors
We partner with leaders in healthcare to understand their needs, and the needs of their patients, so we can develop new product solutions that will improve their lives.

Dr. Sergey V. Kantsevoy
Professor of Medicine, Director of Therapeutic Endoscopy, Mercy Medical Center

Emre Gorgun, MD
Department of Colorectal Surgery, The Cleveland Clinic

David L. Diehl, MD
Director of Interventional Endoscopy, Geisinger Medical Center

Mohamed O. Othman, MD
Chief of Gastroenterology Section, Baylor College of Medicine