Lumendi shines at TxIEG 2025, a record-breaking year for the company with unprecedented interest in the DiLumen Endoluminal Interventional Platform.
Louis Gutierrez, Clinical & Regional Sales Manager attends the Cedar-Sinai International Symposium


Lumendi shines at TxIEG 2025, a record-breaking year for the company with unprecedented interest in the DiLumen Endoluminal Interventional Platform.
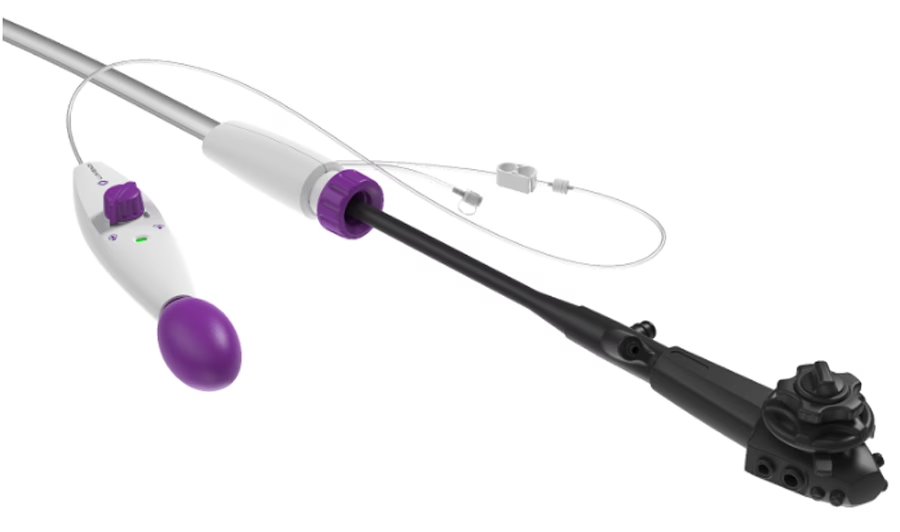
“This certification is a key milestone in our global strategy to expand access to minimally invasive endoluminal procedures,” stated Peter Johann, Ph.D., Executive Chairman of Lumendi AG.