Lumendi shines at TxIEG 2025, a record-breaking year for the company with unprecedented interest in the DiLumen Endoluminal Interventional Platform.
Louis Gutierrez, Clinical & Regional Sales Manager attends the Cedar-Sinai International Symposium


Lumendi shines at TxIEG 2025, a record-breaking year for the company with unprecedented interest in the DiLumen Endoluminal Interventional Platform.
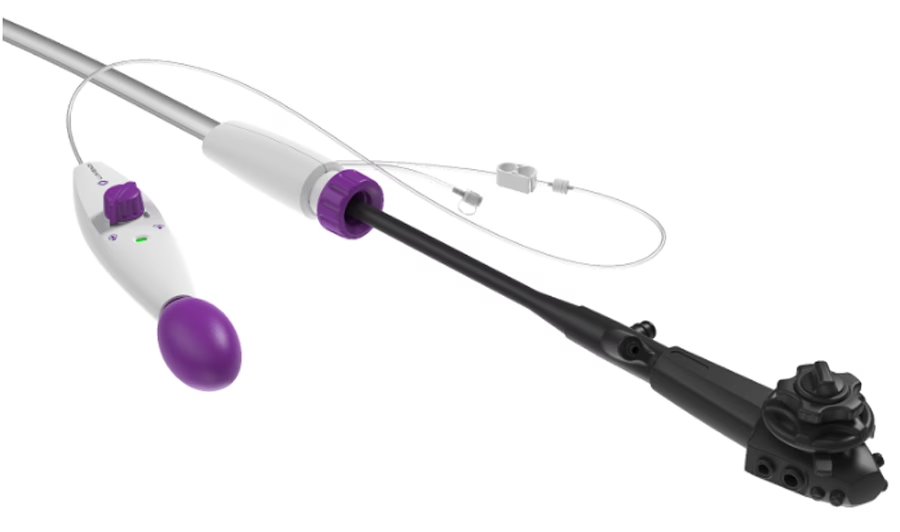
“This certification is a key milestone in our global strategy to expand access to minimally invasive endoluminal procedures,” stated Peter Johann, Ph.D., Executive Chairman of Lumendi AG.
Endoscopic removal of colonic polyps and early cancers eliminates the need for surgical resection and possible temporary or permanent colostomy.