Untitled

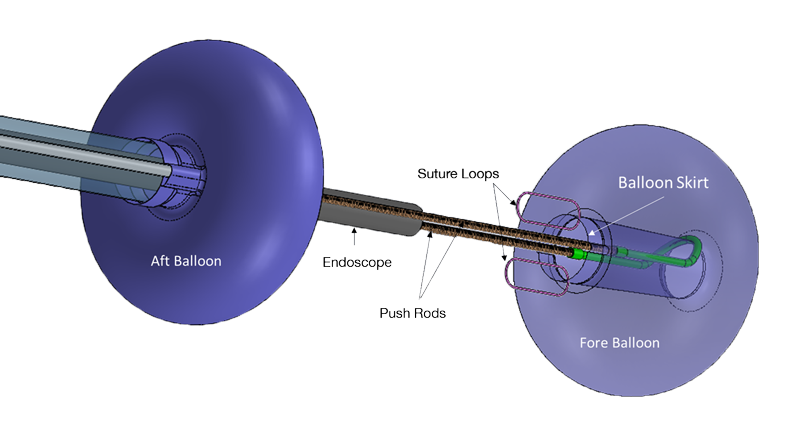
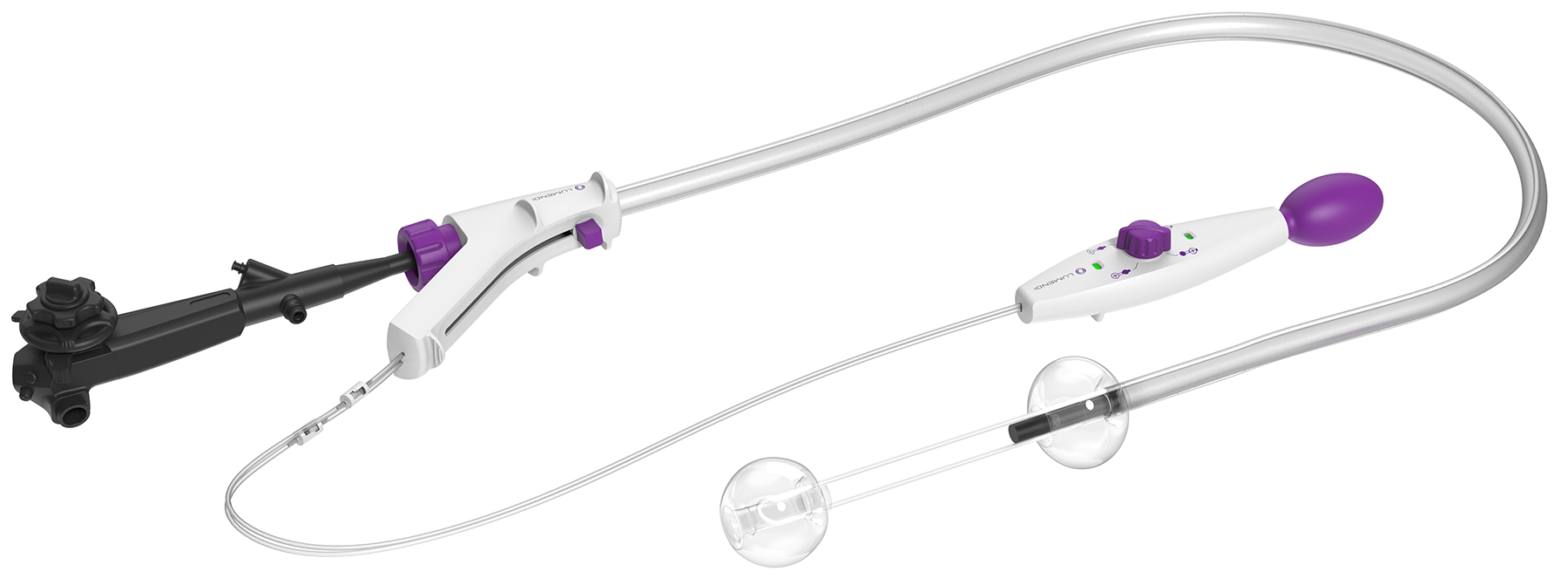
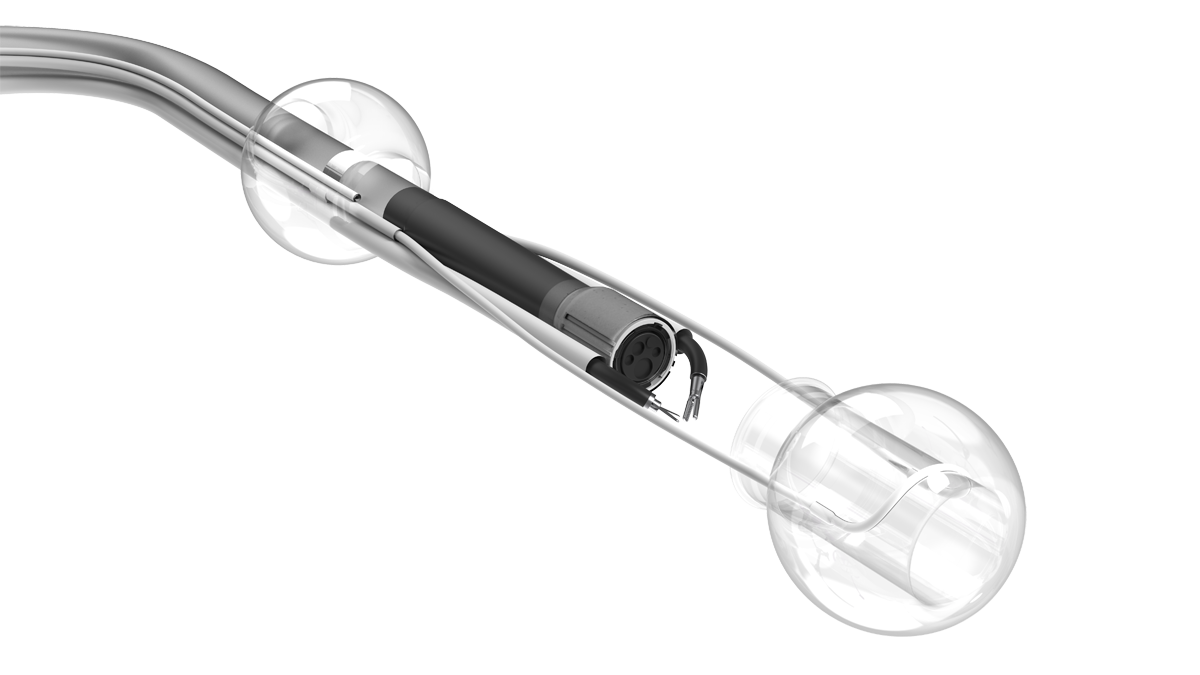
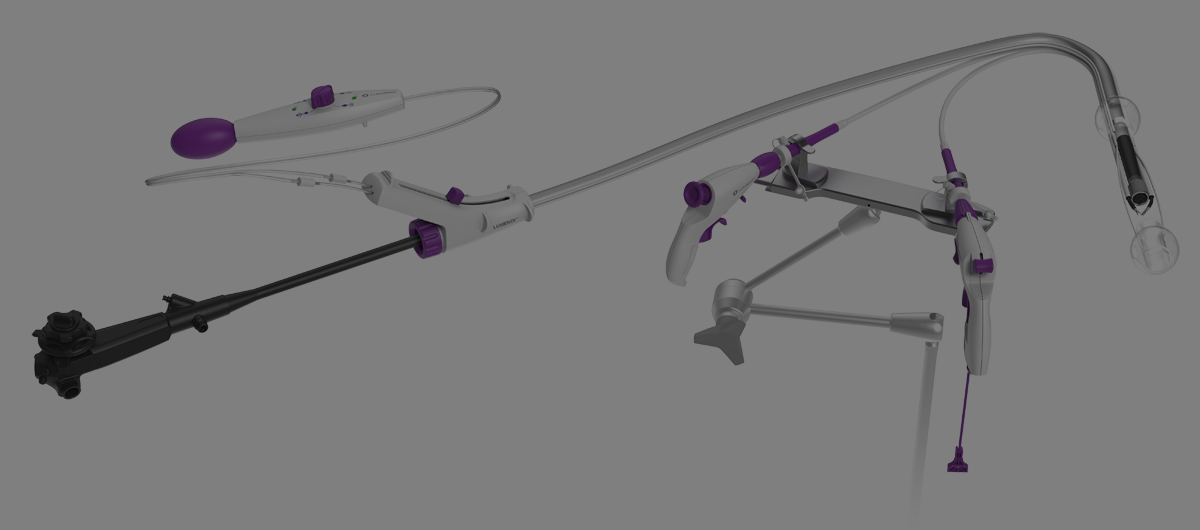
LUMENDI RECEIVES CE MARK FOR DILUMEN™ EZ¹ AND DILUMEN™ C¹, EXPANDING FOOTPRINT IN THE EUROPEAN MARKET ZUG, Switzerland, Oct. 29, 2025 /PRNewswire/ Lumendi AG, a leader in advanced endoluminal technologies, today… Continue reading Untitled