FLEXIBLE ENDOSCOPIC DOUBLE BALLOON PLATFORM: FROM CONCEPT TO HUMAN FEASIBILITY TRIAL
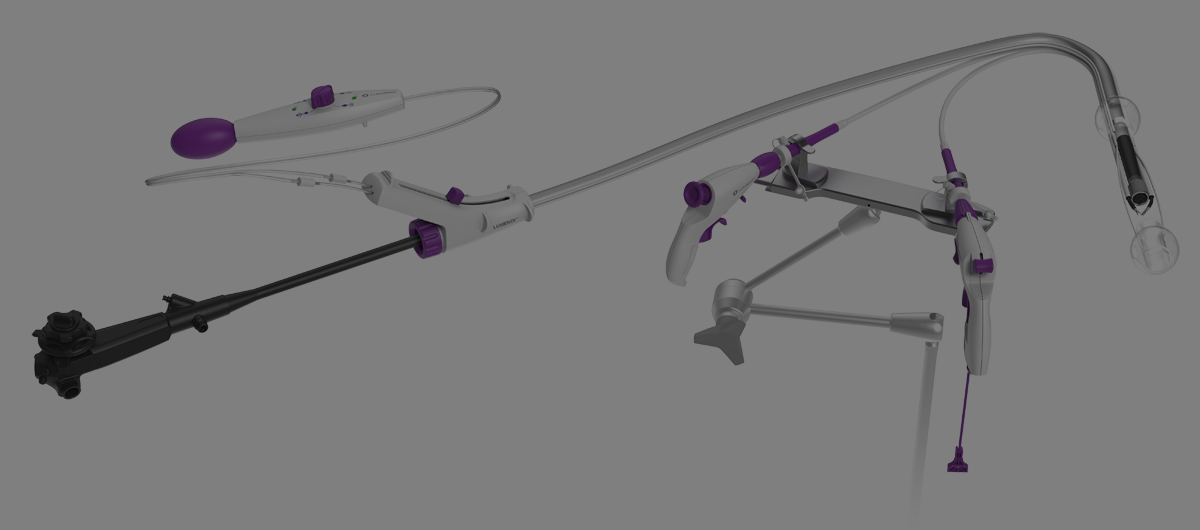
Flexible Endoscopic Double Balloon Platform: from concept to human feasibility trial. Sam Sharma*, Kota Momose, Toyooki Sonoda, Reem Z. Sharaiha Weill Cornell Medical College, New York, NY Background: Despite the… Continue reading FLEXIBLE ENDOSCOPIC DOUBLE BALLOON PLATFORM: FROM CONCEPT TO HUMAN FEASIBILITY TRIAL